Hi,
I have a naive question here.
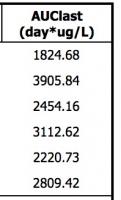
We want to do an NCA analysis with multiple dosing at Day 0, 7, and 14 during a 21-day Cycle 1. I know a dosing record is not mandatory when calculating AUC. However, my PK parameters (AUClast) turned out to be different when I left dosing empty comparing when I input a multi-dosing record (calculation results in the attachment section). Meanwhile, R gave me the same results with the one without dosing record in Pheonix WNL.
I am wondering why this can happen and I still want to somehow find a way to include dosing since a clearance is required for the analyses.
Thank you guys!