Hi John,
I saw the discussion at David’s list and had no time to answer over there yet. I don’t concur with the majority (as usual) and strongly support your idea of using
predicted values. It is my standard for 35 years and I never received a request from any agency in my 500+ studies so far. ;-)
Two references recommending the extrapolation based on C
pred/λ
z (instead of C
obs/λ
z):
- Sauter R, Steinijans VW, Diletti E, Böhm E, Schulz H-U
Presentation of results from bioequivalence studies
Int J Clin Pharm Ther Toxicol 30/Suppl.1, S7–30 (1992) - Hauschke D, Steinijans VW, Pigeot I
Bioequivalence studies in drug development methods and applications
John Wiley & Sons, New York (2007)
The FDA and the WHO recommend [
sic] C
obs/λ
z, but as usual nonbinding, blahblah. No specific method mentioned by the EMA. If you have a lot of spare time you can check my collection:
http://bebac.at/Guidelines.htm
BTW, AUC
∞ is a lousy metric for the extent of absorption. Quote from the abstract of an antique paper (some of the authors the usual suspects from the FDA):
The accuracy, precision, and ease of use of the various measures of extent were evaluated, and statistical power analyses were performed. Among the measures tested, the most reliable were the AUC computed up to the time of the last quantifiable concentration, without extrapolation, and Cmax.
(my emphasis)
Bois FY, Tozer TN, Hauck WW, Chen M-L, Patnaik R, Williams RL
Bioequivalence: Performance of Several Measures of Extent of Absorption
Pharm Res 11(5), 715–22 (1994)
If you read the paper in all of its doubtful beauty, you will see that AUC
∞ is not only more variable than AUC
t (trivial), but might also be biased (depended on the number of “true” compartments and the LLOQ). I never understood why the FDA requires it at all. We know that the last observed concentration (since close to the LLOQ) likely is the most inaccurate and imprecise point of the entire profile. If we base the extrapolation on this value, all its variability will be carried over to AUC
∞. Not a particularly good idea.
Of course my views are biased since my background is (human) BA/BE. In preclinical studies / TK ruled by GLP AUC
∞ is important indeed.
I also suggest to fiddle around in PHX in order to get C
pred in the output. Steps:
- Send the NCA Final Parameters Pivoted to the DW
- Exclude everything you will not need for sorting (subject, treatment, etc) and the irrelevant columns. Keep at least Tlast and Clast (if you want to check whether the calculation is correct, keep lambda_z, AUClast, and AUCpred as well).
- In the Properties change Tlast to time.
- Send the Result to Join Worksheets
- Worksheet 1: Sort ad libitum; time is mandatory
- Map whatever you need to Source
- Worksheet 2: Summary Table of NCA, same sorts as above + time.
- Check “Inner Join” (important!)
- Here you are.
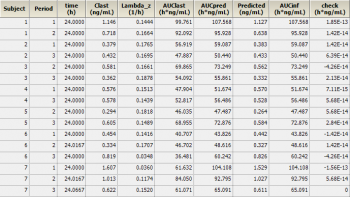
If you don’t trust PHX, you can add another transformation and calculate AUCinf as Predicted/lambda_z and compare it with AUCpred. In my validation runs I never saw any deviation larger than 2E-13 (see screenshot) ;-)
I have C
pred in my standard reports to make the life of reviewers easier. I would love if Certara/Pharsights adds C
pred the NCA-output…
A final remark: It’s necessary to have an SOP, but in my experience it is better to be as transparent as possible. State in the study protocol exactly what you will do – not only refer to an SOP. The protocol is reviewed and approved by the agency. Avoids discussions in the end.
Good luck,
Helmut
Nitpicking PS: The EMEA changed its name to EMA in 2005.
Edited by Helmut Schütz, 27 November 2015 - 07:58 PM.